NOx
Nitrogen oxides appear in many variationa: the most commons ones are NO, NO2 N2O, N2O3, N2O4 or N2O5. From which only the first three are stable at atmospheric conditions. Combustion processes form mostly NO and NO2. This mixture is summarized under the generic term NOx. The typical NO2 fraction of NOx is normally in the order of 5%, thus only the NO concentration is measured and the NO2 content is compensated by a correction factor.
NOx formation and destruction
The reaction mechanism is studied since the last century and large progress was made, especially the progress in simulating reactions was significant. Today three mechanism of NOx formation in combustion processes were identified:
- thermal NO
- fuel NO
- prompt NO
The thermal NO formation can be described by the Zeldovich-mechanism:
N2 + O → NO + N
N + O2 → NO + O
N + OH → NO + H
The reaction rate are strongly temperature depending a thus the thermal NO formation mechanism is predomintant at temperatures above 1400 °C.
The so called Fuel-NO is formed by oxidation of the fuel bounded nitrogen and is thus minor important for gaseous fuels. Especially at oil or specific coal firing a significant amount of NO can be formed from the fuel. A prevention of forming NO from the fuel is hardly possible.
The prompt NO formation mechanism describes the NO formation in fuel rich rich regions. The reation rate is very fast with a weak temperature relation. Based on the local radical concentration the prompt NO-mechanism can react backwards and can destroy NO.
The NO concentration in the flue gas is always a result of NO formation and destruction. The actual value depends on the local temperature and species distribution. In general following assumption can be drawn:
- Lower temperature give lower NO concentrations
- Lower O2 concentration results in lower NO concentrations
Primary Measures
Based on the discovery of the temperature and concentration field dependence of the NO formation many technical solutions were developed. The basic idea behind was to reduce the peak temperature and Oxygen concentration to prevent the NO formation by the thermal NO mechanism.
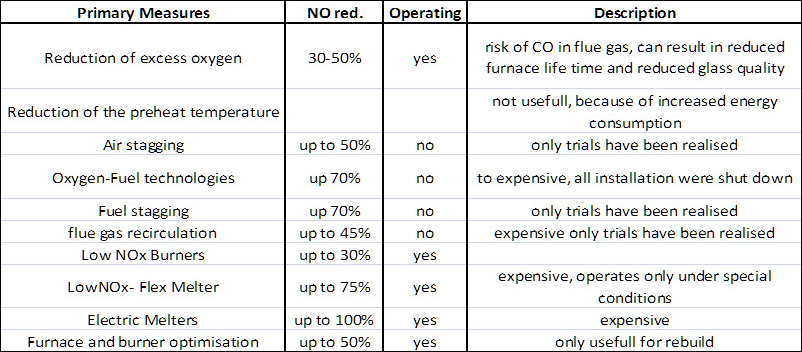
Figure 1: Overview of most common primary methods for NOx reduction in glass melting furnaces
The most effective and most used approach was to manipulate the mixing of fuel and the oxidiser and results in new so called Low-NOx-Burner. Later the NOx formation was reduced by enhancing the recirculation of burned flue gas into the flame to reduce both the peal temperature and the oxygen concentration. Another very effective method to reduce temeperature and concnetration was to stage the fuel or oxidizer supply.
Identifying the best available solution for a furnace is not as easy as Figure 1 implies. A common state of the Art regenerative end-port furance operates at NOx emissions of 650 mg NOx /Nm³. Lower values can be only reached by increasing the CO emissions and the related risk for the regenerator or by reducing the specific melting rate and increasing the cullet fraction.
A solution for further NOx emissions reduction, is the ggc technolgy ggENox.
Secondary Measures
In cases state of the art primary measures are not sufficient to reach the demanded emission limits, secondary measures are necessary to clean the flue gas.
Today three different technologies are used: reburn systems, SNCR or SCR.
The reburning system injects fuel into the regenerators to reburn the NOx back to pure nitrogen. The advantages are low investment cost, which are paid by large operating costs and an increased corrosion of the checkers.
Selective non catalytic reaction (SNCR) and selective catalytic reachtion (SCR) systeme using urea or ammonia to reduce the NOx to nitrogen. The differences are the catalytic enhence of the reactions. Bost systems require large investments and significant operation cost.
The disadvantages of all secondary measures are large investment costs and compoarable high operating costs. The reduction potential is given with up to 80%, which is normally sufficient to reach NOx emissions below 500 mg /Nm³, dry, related to 8% Oxygen. Thus the installation of a secondary measure is avoided where possible.
Today those occasions are greenfield project or large plants, which are forced by government to emit less than 500 mg /Nm³, dry, related to 8% Oxygen.
With our new technology ggENox in many cases a secondary measure can be avoided or at least the costs can be reduced dramatically.
Our Ideas
We follow the simple concept that avoiding NOx formation is better than later correction.
Also the well known remarks “The Solution for Pollution is Dillution.” is still a usefull approach.
All of our ideas can be summaried by the two sentences above. We reach those targets by enhencing the already in every furnace present reduction mechanisms by adjusting the main firing and inserting additional lances and burners like ggENOX.
Further improvements can be realisied by changing significant parts of the combustion into a flameless (other names are MILD or hitac) combustion. We conducted several studies to check the feasibility of those concepts and to evaluate the effect on NOx reduction, fuel consumption, glass quality and maximum possible pull rates (for more information click here)

